Cardiovascular
PAINE PANCE Postcard – Kawasaki Disease

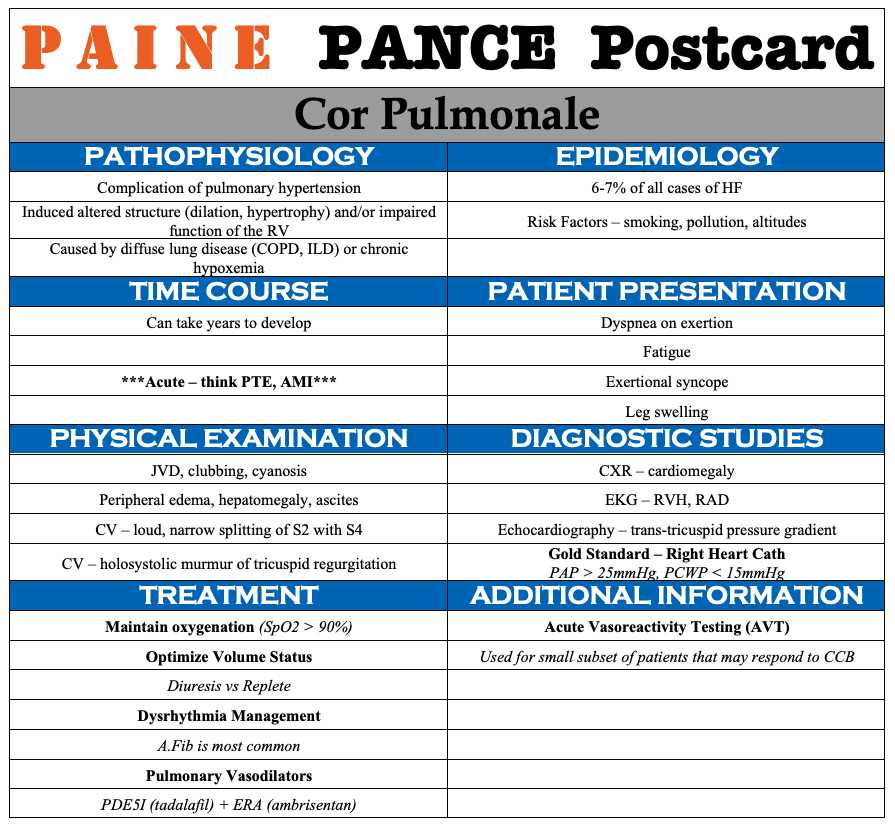
Cardiovascular – Cardiogenic Shock

Cardiovascular – AV Blocks

Cardiovascular – Sinus Node Dysfunction
***LISTEN TO THE PODCAST HERE***
Ep-PAINE-nym
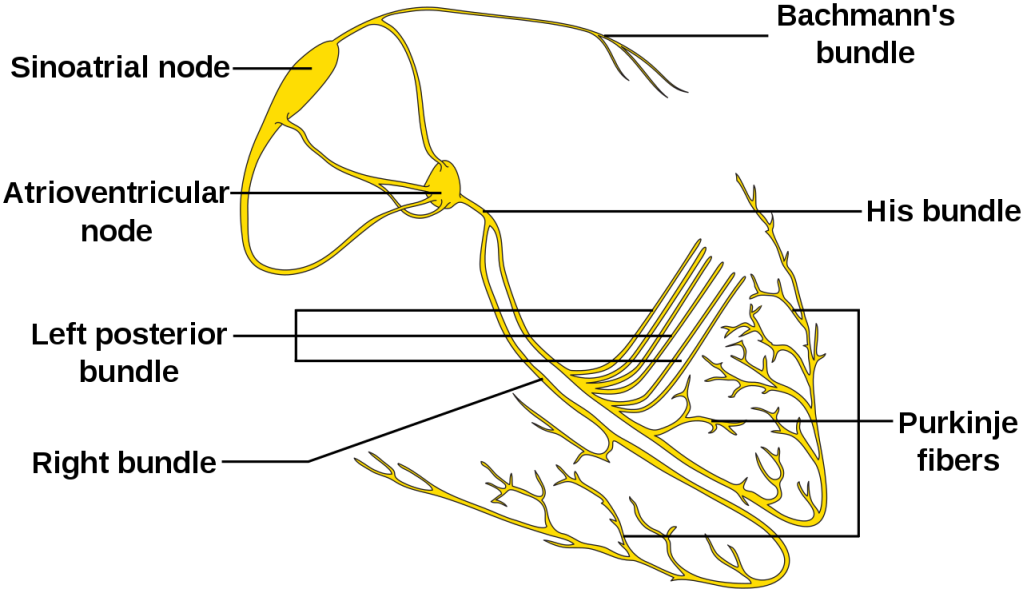
Purkinje Fibers
Other Known Aliases – subendocardial branches
Definition – specialized conducting fibers composed of electrically excitable cells located just beneath the subendocardium in the inner ventricular walls of the heart

Clinical Significance – these cells actually conduct cardiac action potentials faster and more effeciently than any other cells in the heart and are responsible for the synchronized contractions of the ventricles during depolarization. They also have intrinsic pacemaking ability at 20-40 bpm to act as a back-up pacing system.
History – Named after Jan Evangelista Purkinje (1787-1869), a Czech anatomist and experimental physiologist who received his medical doctorate from Charles University in Prague in 1818. He would be appointed Professor of Physiology in Breslau in 1823 and did revolutionary work on vision. He would later create the world’s first Department of Physiology at the University of Breslau in Prussia in 1839 and the world’s second official physiology lab in 1842. During his career of physiologic discovery and research, he discovered large neurons with branching dendrites in the cerebellum (Purkinje cells), describe the change in brightness of red and blue colors as light intensity decreases (Purkinje shift), and the eye’s reduced sensitivity to dim red light compared to dim blue light (Purkinje effect). He also was the first scientist to present work on the cellular theory of biology, the first to use the term “protoplasm” to describe the fluid in cells, and the first to report on the individuality of fingerprints. But it was in 1839 when he described his eponymous fibers of the heart. He was one of the best known scientists of his era and was so famous, people would address letters to him and simply put “Purkinje, Europe”.

References
- Firkin BG and Whitwirth JA. Dictionary of Medical Eponyms. 2nd ed. New York, NY; Parthenon Publishing Group. 1996.
- Bartolucci S, Forbis P. Stedman’s Medical Eponyms. 2nd ed. Baltimore, MD; LWW. 2005.
- Yee AJ, Pfiffner P. (2012). Medical Eponyms (Version 1.4.2) [Mobile Application Software]. Retrieved http://itunes.apple.com.
- Whonamedit – dictionary of medical eponyms. http://www.whonamedit.com
- Up To Date. www.uptodate.com
- Cavero I, Guillon JM, Holzgrefe HH. Reminiscing about Jan Evangelista Purkinje: a pioneer of modern experimental physiology. Adv Physiol Educ. 2017; 41(4):528-538. [pubmed]
PAINE #PANCE Pearl – Cardiovascular
Question
74yo woman, with a history of CAD and hyperlipidemia, presents to your office with a 6-month history of leg pain and swelling. She states that it seems to be worse when she is on her feet and improves when she can put her legs up. She denies worsening pain with activity or walking, but has recently developed a “rash” on her legs that is worrying her (see below). Physical examination reveals warmth to the feet and legs with scattered, thin hair. You appreciate 1+ DP and PT pulses bilaterally.
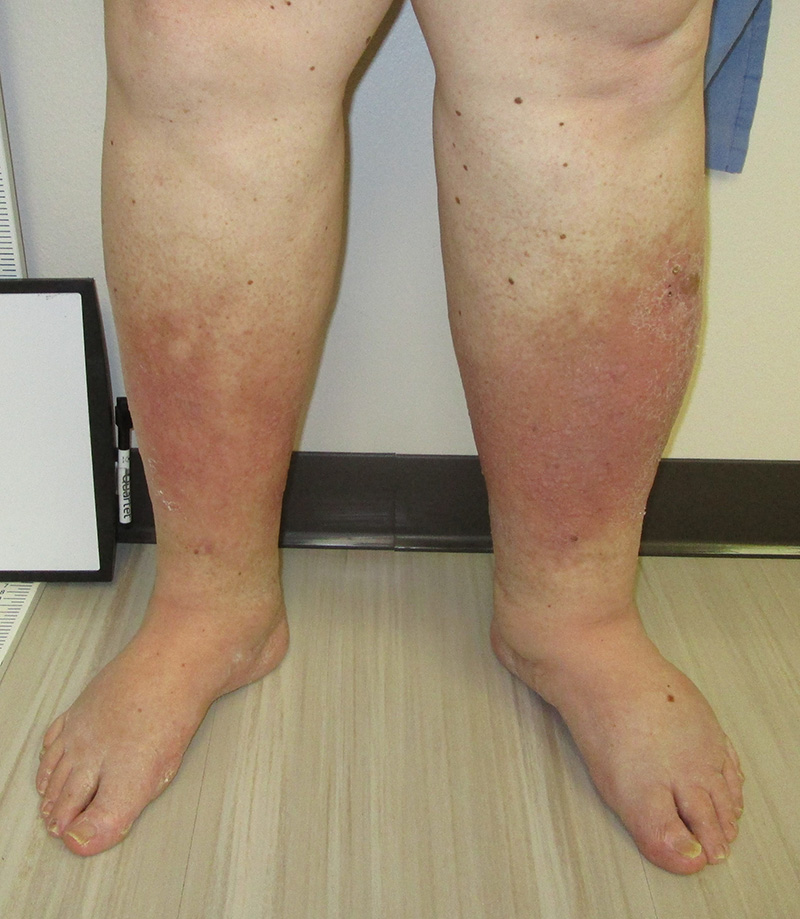
- What are some bedside maneuvers or tests you can perform to differentiate between arterial and venous insufficiency?
- What are the findings associated with each?
Ep-PAINE-nym
Bundle of His
Other Known Aliases – atrioventricular bundle
Definition – collection of electrical conduction cells of the heart that transmit impulses from the AV node to the ventricles
Clinical Significance – this bundle of cells is responsible for communication contraction impulses from the atria to the ventricles. Any damage to this area can result in varying degrees of heart block and conduction abnormalities
History – Named after Wilhelm His Jr. (1863-1934), a Swiss-born cardiologist and anatomist who received his medical doctorate from the University of Leipzig in 1889. The son of the equally famous Basel anatomist Wilhelm His Sr., he would become professor extraordinaire at his alma mater 6 year after graduating. He also went on to be physician-in-chief at the Friedrichstadt Hospital in Dresden, chair of internal medicine in Berlin, and advisory internist for several armies during World War I. He would describe his eponymous bundle as an assistant professor in 1893.

References
- Firkin BG and Whitwirth JA. Dictionary of Medical Eponyms. 2nd ed. New York, NY; Parthenon Publishing Group. 1996.
- Bartolucci S, Forbis P. Stedman’s Medical Eponyms. 2nd ed. Baltimore, MD; LWW. 2005.
- Yee AJ, Pfiffner P. (2012). Medical Eponyms (Version 1.4.2) [Mobile Application Software]. Retrieved http://itunes.apple.com.
- Whonamedit – dictionary of medical eponyms. http://www.whonamedit.com
- Up To Date. www.uptodate.com
- His Jr, W. Die Tätigkeit des embryonalen Herzens und deren Bedeutung für die Lehre von der Hezbewegung beim Menschen. Arbeiten aus der medidizinischen Klinik zu Leipzig, 1893: 23.
#68 – Bundle Branch Blocks
***LISTEN TO THE PODCAST HERE***
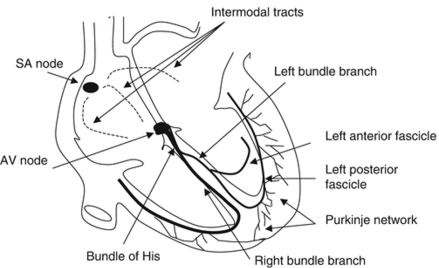
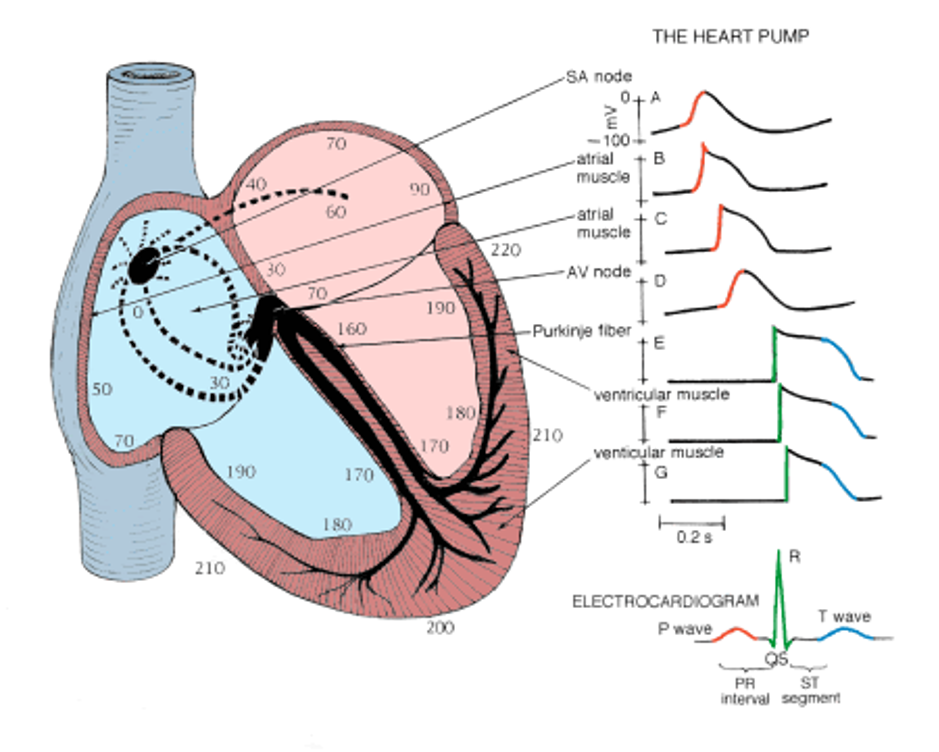
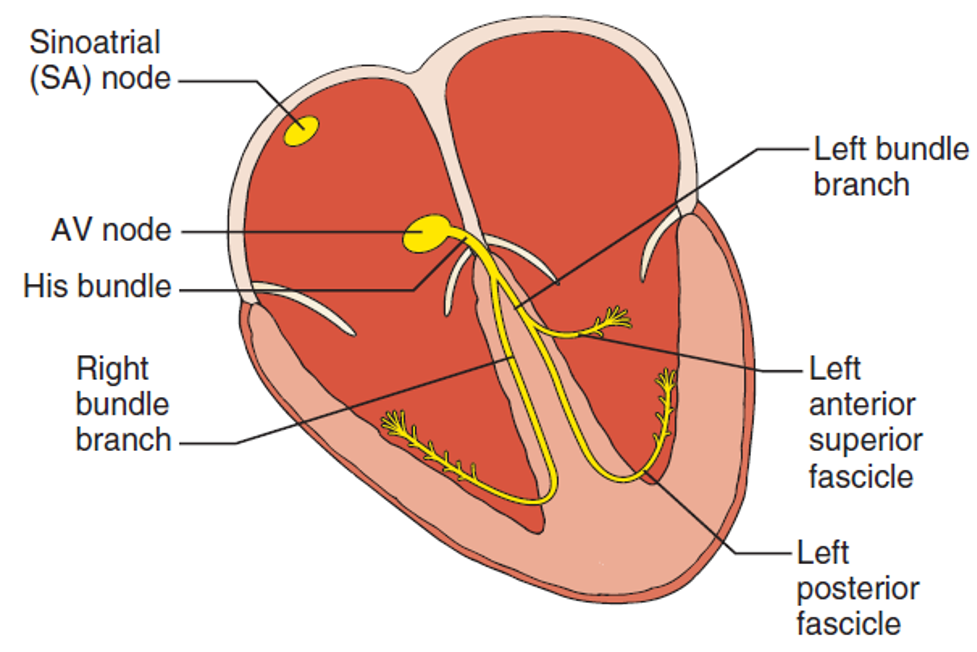
Review of the Electrical Conduction System of the Heart
- Cardiac cells specialized to initiate and distribute electrical impulses in an orderly and sequential manner
- Sinoatrial Node
- Located in superior region of the crista terminalis
- SVC feeds into the right atrium
- Pacemaker of the heart and initiates the heartbeat
- Starts in the node, spreads down the walls of the atria, until it reaches the AV node, stimulating contraction of the myocardium
- Arterial Supply – SA nodal artery via the RCA
- Located in superior region of the crista terminalis
- Atrioventricular Node
- Located in interatrial septum above the coronary sinus near the attachment of the septal cusp of the tricuspid valve (Triangle of Koch)
- Passes SA node impulse to the AV bundle (Bundle of His)
- Arterial Supply – AV nodal artery via the RCA (80-90%) or LCx (10-20%)
- AV Bundle (Bundle of His)
- Arises from the AV node and descends along the membranous portion of the interventricular septum, where it divides at the upper border of the muscular portion of the interventricular septum into the left and right bundles suppling their respective ventricles
- Transmits AV nodal impulses through the interventricular septum to the left and right bundle branches, which gives rise to the Purkinje fibers that ultimately distribute the ventricular myocardium
Bundle Branch Blocks Pearls
- R-wave = depolarization going TOWARDS the lead
- S-wave = depolarization going AWAY from the lead
- RBBB = delay in conduction is oriented to the RIGHT and ANTERIOR
- QRS = Positive V1 and negative V6
- LBBB = delay in conduction is oriented to the LEFT and either ANTERIOR or POSTERIOR
- QRS = Negative V1 and positive V6
- Wide QRS complex > 120 ms
- Delay in conduction due to the block
- Secondary Repolarization (ST-T) Abnormalities
- T-wave discordance with last deflection of QRS
Causes
- RBBB
- More common in patients without structural heart disease
- Congenital
- ASD
- Cardiac
- Valvulopathies, ischemic heart disease
- Pulmonary
- Pulmonary HTN, PTE
- LBBB
- 4 main underlying conditions
- Coronary disease
- Hypertensive heart disease
- Aortic valve disease
- Cardiomyopathies
- 4 main underlying conditions
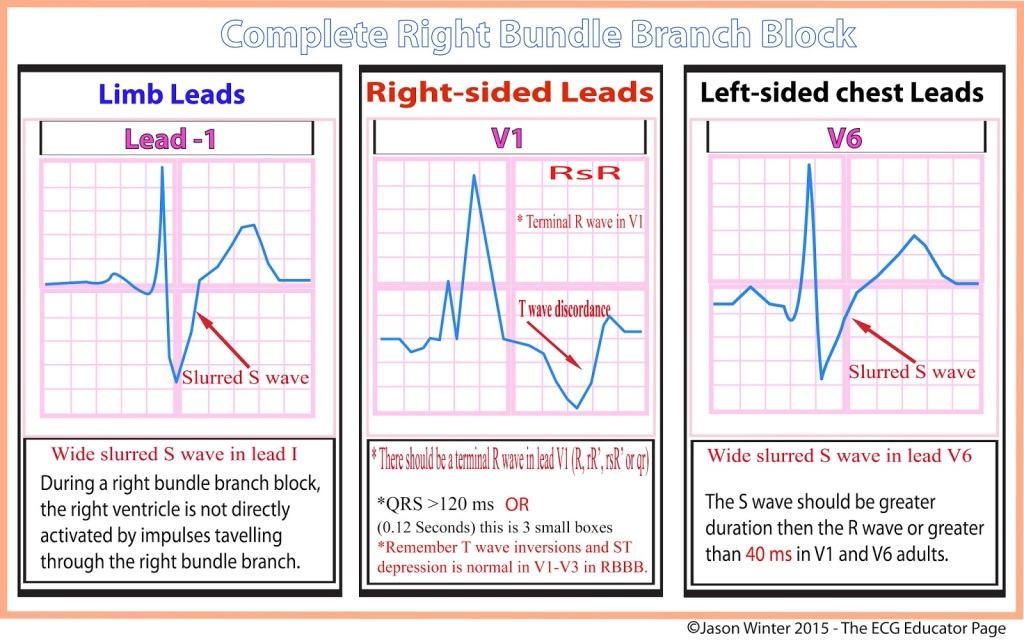
RBBB EKG Diagnostic Criteria
- QRS > 120 ms
- If all other criteria met but QRS < 120 ms, it is termed incomplete RBBB
- rSR’ pattern in V1 and V2
- Slurred S-wave in lateral leads (I, aVL, V5, V6)
- ST depression and T-wave inversion in V1-V3

LBBB EKG Diagnostic Criteria
- QRS > 120 ms
- Dominant S-wave in V1-V3
- Moving away from the leads
- Broad, monophasic (M-shaped or notched) R-wave in lateral leads (I, aVL, V5, V6)
- Moving towards the leads
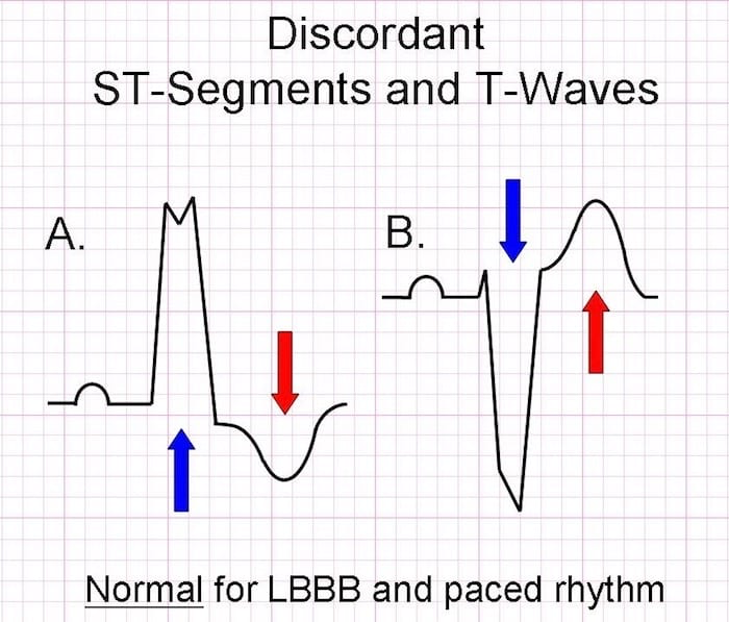
- Appropriate discordance
- ST-segment and T-wave are in OPPOSITE direction to the main vector of the QRS complex
- Left axis deviation
- Poor R-wave progression
SPECIAL CONSIDERATIONS
Fascicular Blocks
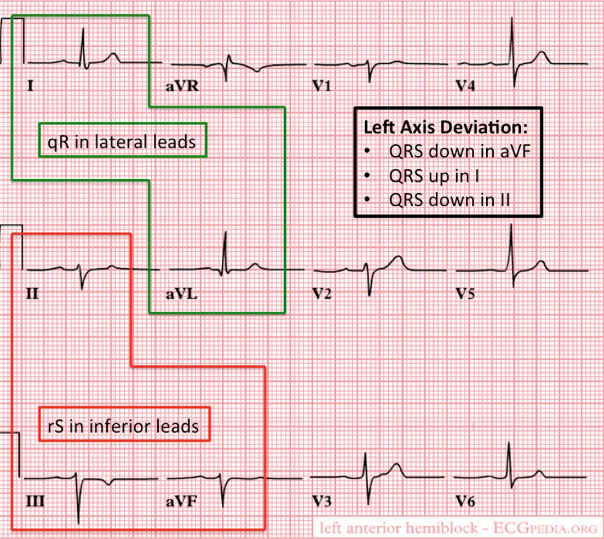
- Anterior
- Pathology
- When blocked, the conduction to the high lateral portion of the ventricle is delayed
- Spreads to the intact posterior fascicle and RBB
- Causes left axis deviation
- Spreads to the intact posterior fascicle and RBB
- When blocked, the conduction to the high lateral portion of the ventricle is delayed
- Criteria for left anterior fascicular block
- QRS normal to slightly prolonged
- Left axis deviation WITHOUT other reasons
- Small R-wave and large S-wave in inferior leads (II, III, aVF)
- Small Q-wave with large R-wave in lateral leads (I, aVL)
- Pathology
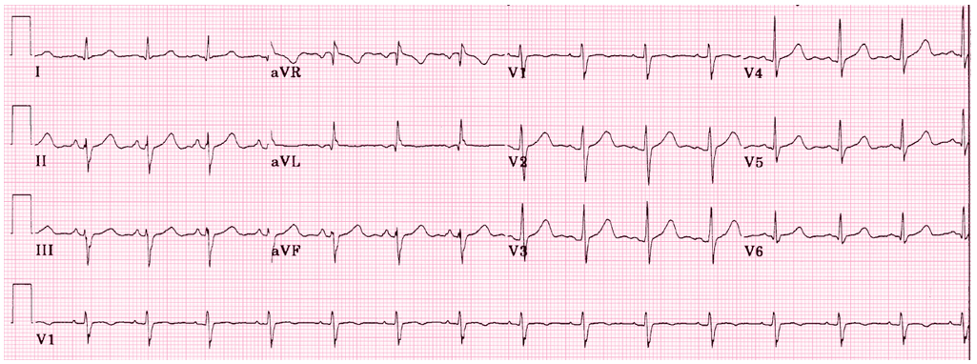
- Posterior
- Pathology
- When blocked, the conduction to the inferior portion of the ventricle is delayed
- Spreads to the intact anterior fascicle and RBB
- Causes right axis deviation
- Spreads to the intact anterior fascicle and RBB
- When blocked, the conduction to the inferior portion of the ventricle is delayed
- Criteria for left posterior fascicular block
- QRS normal to slightly prolonged
- Right axis deviation WITHOUT other reasons
- Small R-wave and large S-wave in lateral leads (I, aVL)
- Small Q-wave and large R-wave in inferior leads (II, III, aVF)
- Pathology
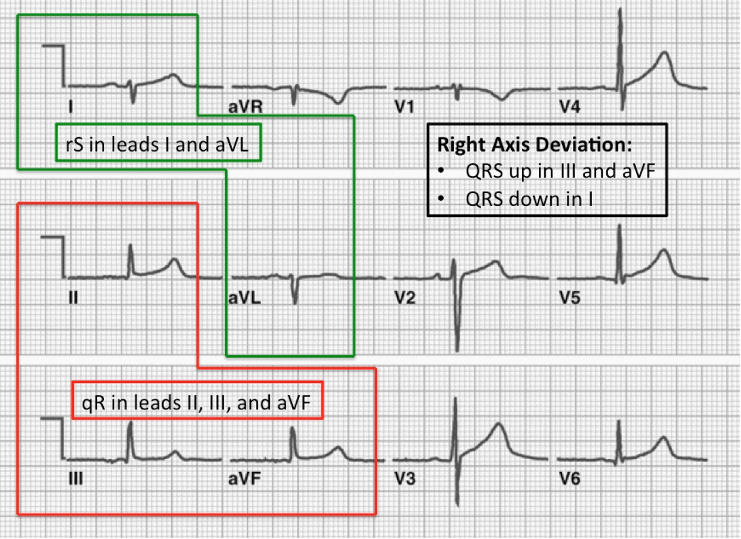
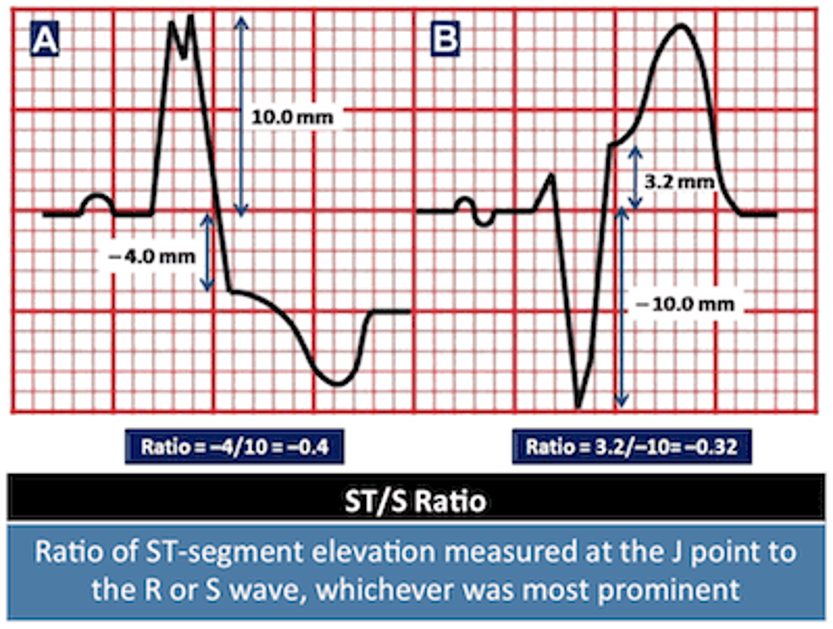
Sgarbossa’s Criteria
- Used in the presence of LBBB or paced rhythm to uncover potential ischemia
- Original (1996)
- Concordant ST elevation > 1mm in leads with a positive QRS complex (5 points)
- Concordant ST depression > 1mm in V1-V3 (3 points)
- Excessively discordant ST elevation > 5mm in leads with a negative QRS complex (2 points)
- Score ≥ 3 has a specificity of 90% for detecting concomitant ischemia
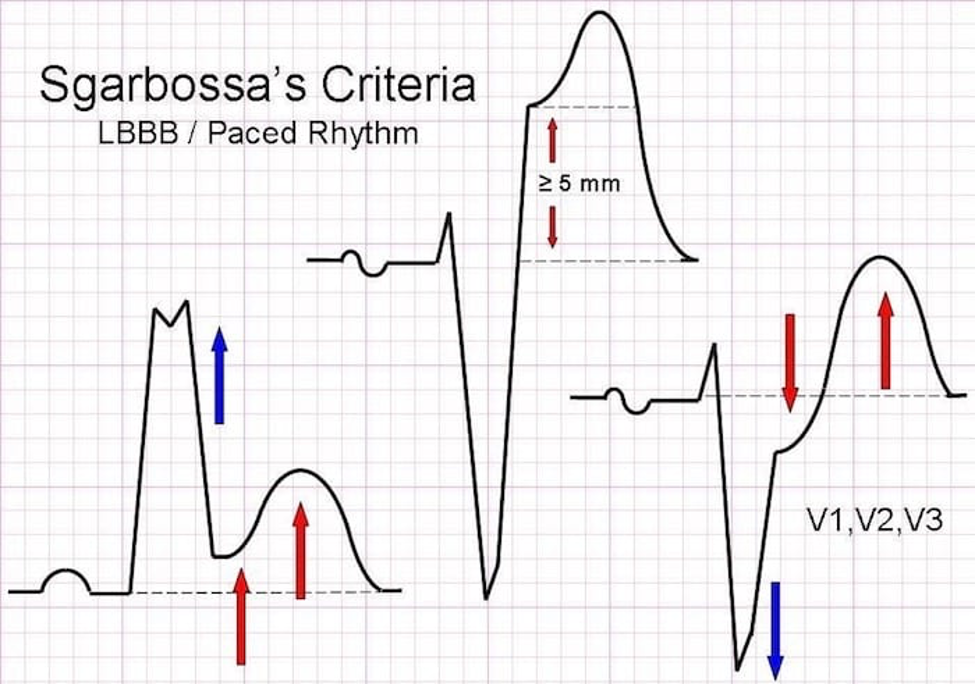
- Smith-Modified Sgarbossa Criteria (2012)
- ≥ 1 lead with ≥ 1mm of concordant ST elevation
- ≥ 1 lead of V1-V3 with ≥ 1mm of concordant ST depression
- ≥ 1 lead ANYWHERE with ≥ 1mm ST elevation AND proportionally excessive discordant ST elevation
- Defined as ≥ 25% of the depth of the preceding S-wave
1893 Cottage Physician

References
- Heart. In: Morton DA, Foreman K, Albertine KH. eds. The Big Picture: Gross Anatomy, 2e. McGraw-Hill; Accessed January 17, 2021.
- Jaffar A. Anatomical Structure of the Heart. In: Elmoselhi A. eds. Cardiology: An Integrated Approach. McGraw-Hill; Accessed January 17, 2021.
- Goldberger AL. Electrocardiography. In: Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J. eds. Harrison’s Principles of Internal Medicine, 20e. McGraw-Hill; Accessed January 17, 2021.
- LITFL. Right bundle branch blocks. https://litfl.com/right-bundle-branch-block-rbbb-ecg-library/
- LITFL. Left bundle branch blocks. https://litfl.com/left-bundle-branch-block-lbbb-ecg-library/
- REBELEM. Bundle Branch Blocks. https://rebelem.com/bundle-branch-blocks101/
- Sgarbossa EB, Pinski SL, Barbagelata A, et al. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. GUSTO-1 (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) Investigators. N Engl J Med. 1996; 334(8):481-7. [pubmed]
- Smith SW, Dodd KW, Henry TD, Dvorak DM, Pearce LA. Diagnosis of ST-elevation myocardial infarction in the presence of left bundle branch block with the ST-elevation to S-wave ratio in a modified Sgarbossa rule. Ann Emerg Med. 2012; 60(6):766-76. [pubmed]
- Meyers HP, Limkakeng AT Jr, Jaffa EJ, et al. Validation of the modified Sgarbossa criteria for acute coronary occlusion in the setting of left bundle branch block: A retrospective case-control study. Am Heart J. 2015; 170(6):1255-64. [pubmed]
- CORE EM. Validation of Modified Sgarbossa Criteria. https://coreem.net/journal-reviews/modified-sgarbossa-criteria/
PAINE #PANCE Pearl – Cardiovascular
Question
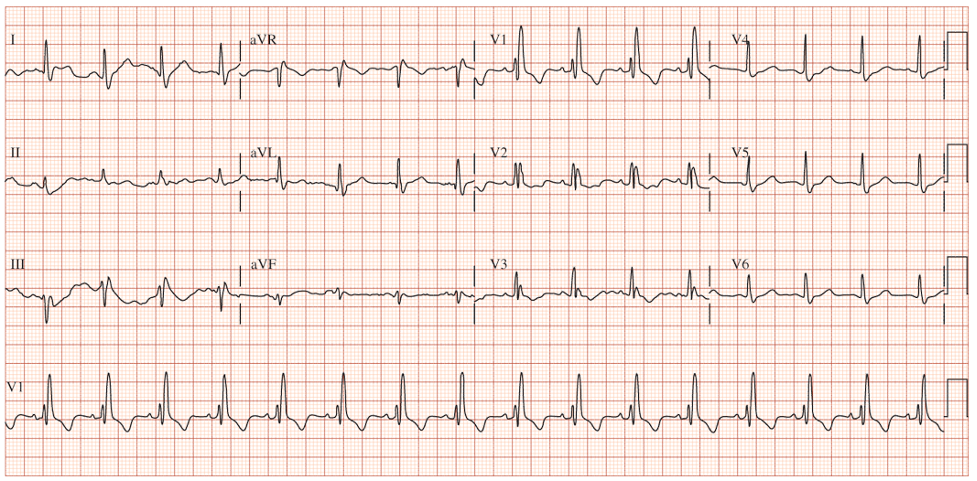
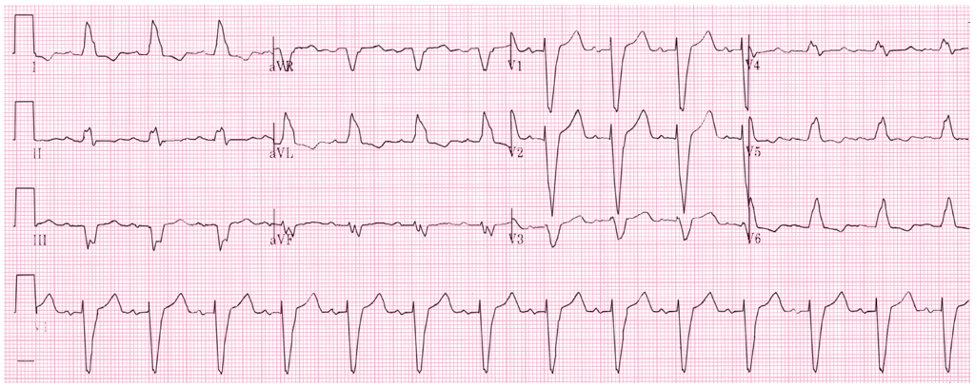
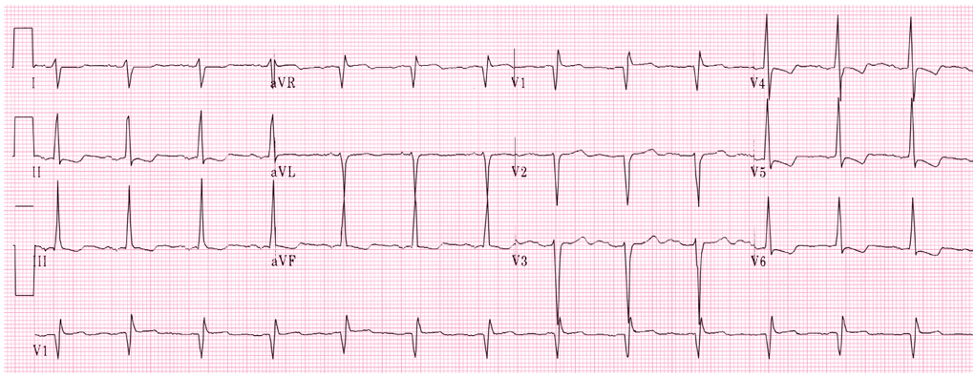
62yo man, with a history of COPD and 52-pack-year history of smoking, presents to your office to establish care. His shortness of breath has been manageable using tiotropium daily with albuterol 2-3x per month for exacerbation. He denies angina, chest pain, or unreasonable dyspnea with exertion. An EKG was performed and is below.
- What does it show?
- What are the diagnostic criteria present?
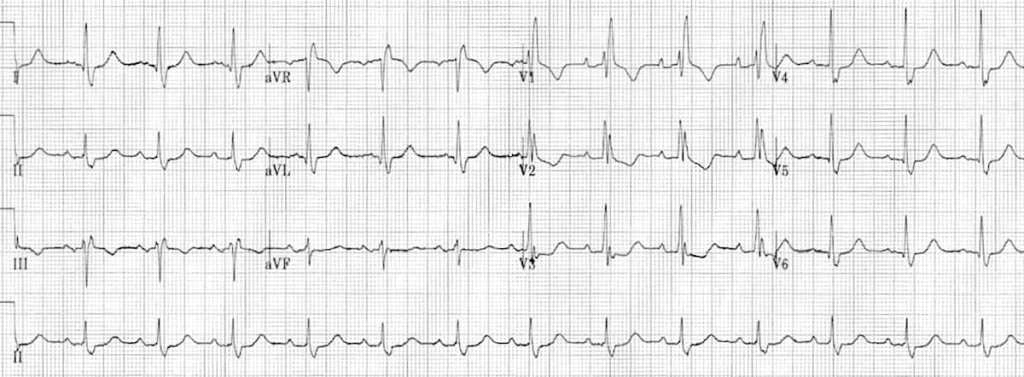
Answer
- The EKG reveals a right bundle branch block most likely due to his underlying COPD and pulmonary hypertension.
- Diagnostic criteria for RBBB are:
- Wide QRS > 120 ms
- RSR’ pattern in V1-2 (“rabbit ears”) with R’ > R
- Wide, slurred S wave in I, aVL, or V5-6
- Other common findings, though not always associated, is ST depression and T wave inversion in the right precordial leads (V1-3)