Emergency Medicine
#68 – Bundle Branch Blocks
***LISTEN TO THE PODCAST HERE***
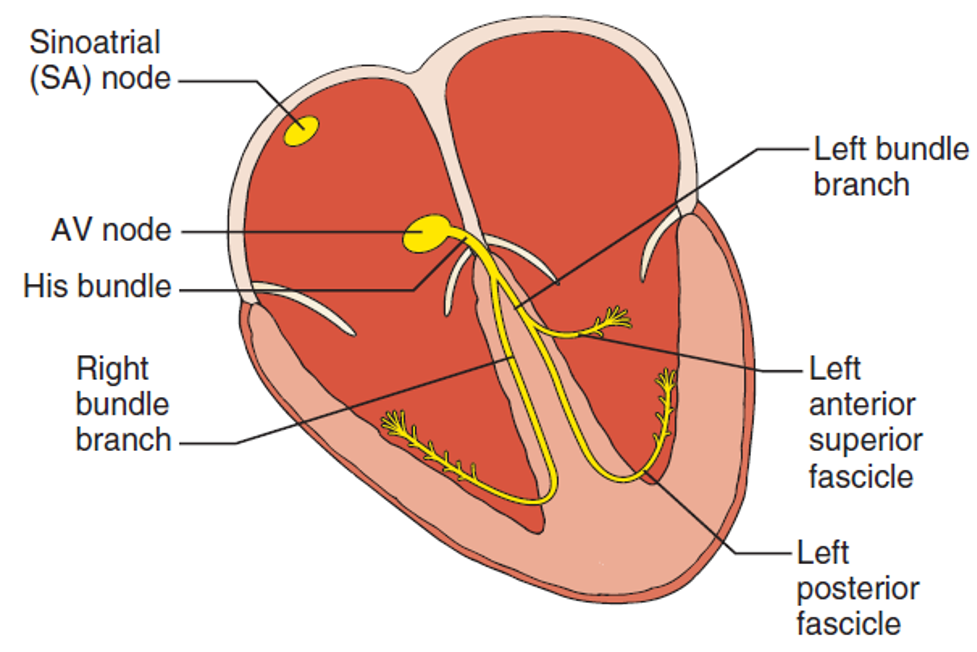
Review of the Electrical Conduction System of the Heart
- Cardiac cells specialized to initiate and distribute electrical impulses in an orderly and sequential manner
- Sinoatrial Node
- Located in superior region of the crista terminalis
- SVC feeds into the right atrium
- Pacemaker of the heart and initiates the heartbeat
- Starts in the node, spreads down the walls of the atria, until it reaches the AV node, stimulating contraction of the myocardium
- Arterial Supply – SA nodal artery via the RCA
- Located in superior region of the crista terminalis
- Atrioventricular Node
- Located in interatrial septum above the coronary sinus near the attachment of the septal cusp of the tricuspid valve (Triangle of Koch)
- Passes SA node impulse to the AV bundle (Bundle of His)
- Arterial Supply – AV nodal artery via the RCA (80-90%) or LCx (10-20%)
- AV Bundle (Bundle of His)
- Arises from the AV node and descends along the membranous portion of the interventricular septum, where it divides at the upper border of the muscular portion of the interventricular septum into the left and right bundles suppling their respective ventricles
- Transmits AV nodal impulses through the interventricular septum to the left and right bundle branches, which gives rise to the Purkinje fibers that ultimately distribute the ventricular myocardium
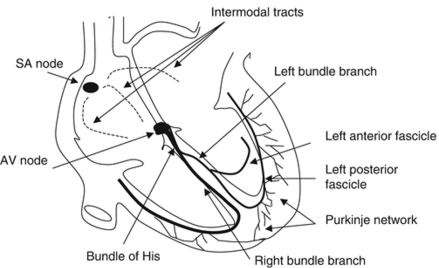
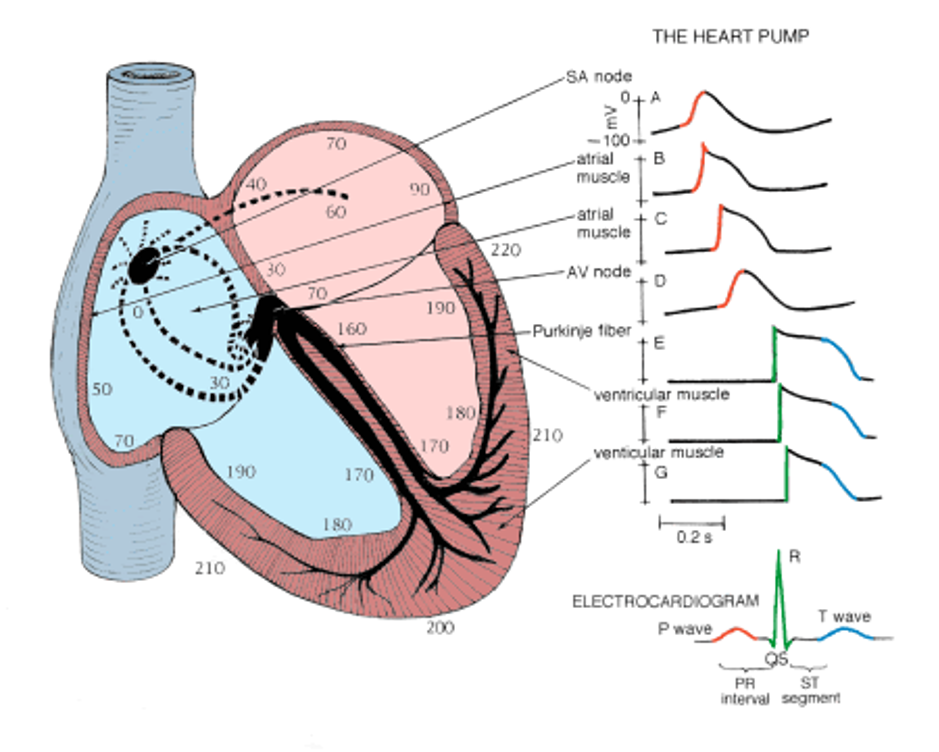
Bundle Branch Blocks Pearls
- R-wave = depolarization going TOWARDS the lead
- S-wave = depolarization going AWAY from the lead
- RBBB = delay in conduction is oriented to the RIGHT and ANTERIOR
- QRS = Positive V1 and negative V6
- LBBB = delay in conduction is oriented to the LEFT and either ANTERIOR or POSTERIOR
- QRS = Negative V1 and positive V6
- Wide QRS complex > 120 ms
- Delay in conduction due to the block
- Secondary Repolarization (ST-T) Abnormalities
- T-wave discordance with last deflection of QRS
Causes
- RBBB
- More common in patients without structural heart disease
- Congenital
- ASD
- Cardiac
- Valvulopathies, ischemic heart disease
- Pulmonary
- Pulmonary HTN, PTE
- LBBB
- 4 main underlying conditions
- Coronary disease
- Hypertensive heart disease
- Aortic valve disease
- Cardiomyopathies
- 4 main underlying conditions
RBBB EKG Diagnostic Criteria
- QRS > 120 ms
- If all other criteria met but QRS < 120 ms, it is termed incomplete RBBB
- rSR’ pattern in V1 and V2
- Slurred S-wave in lateral leads (I, aVL, V5, V6)
- ST depression and T-wave inversion in V1-V3

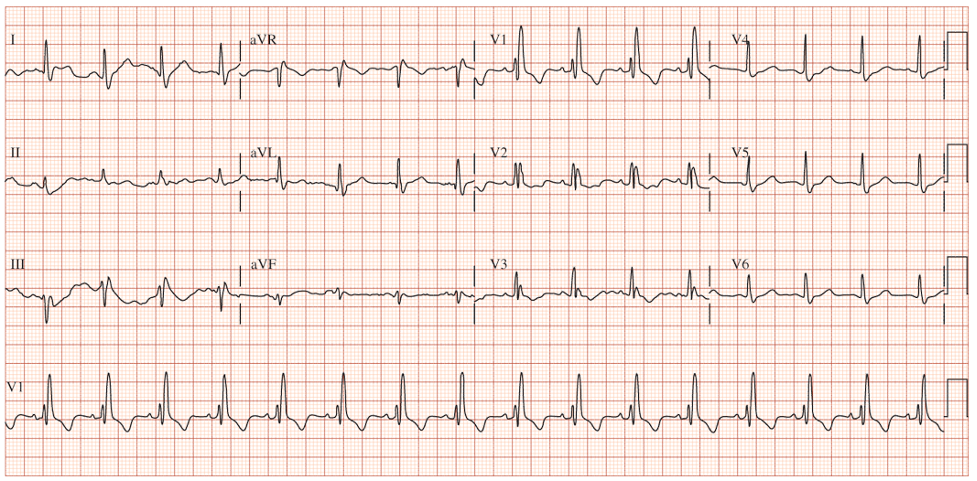
LBBB EKG Diagnostic Criteria
- QRS > 120 ms
- Dominant S-wave in V1-V3
- Moving away from the leads
- Broad, monophasic (M-shaped or notched) R-wave in lateral leads (I, aVL, V5, V6)
- Moving towards the leads
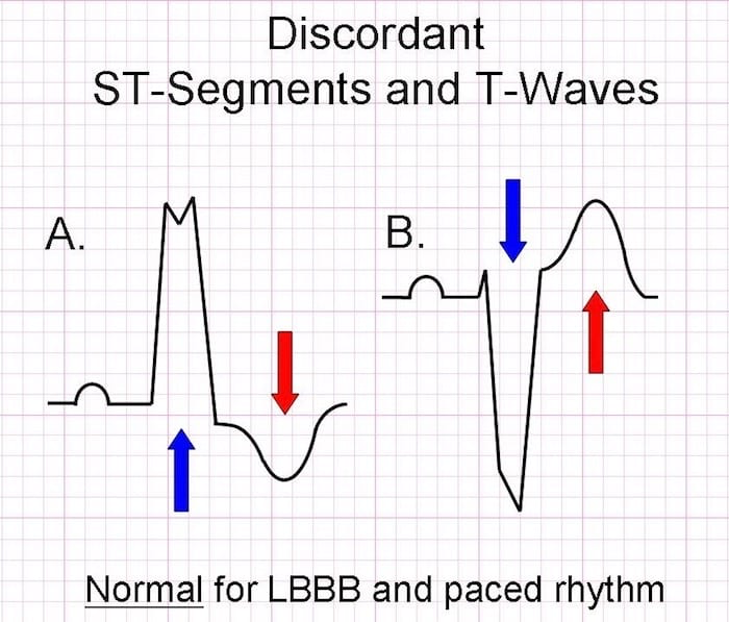
- Appropriate discordance
- ST-segment and T-wave are in OPPOSITE direction to the main vector of the QRS complex
- Left axis deviation
- Poor R-wave progression
SPECIAL CONSIDERATIONS
Fascicular Blocks
- Anterior
- Pathology
- When blocked, the conduction to the high lateral portion of the ventricle is delayed
- Spreads to the intact posterior fascicle and RBB
- Causes left axis deviation
- Spreads to the intact posterior fascicle and RBB
- When blocked, the conduction to the high lateral portion of the ventricle is delayed
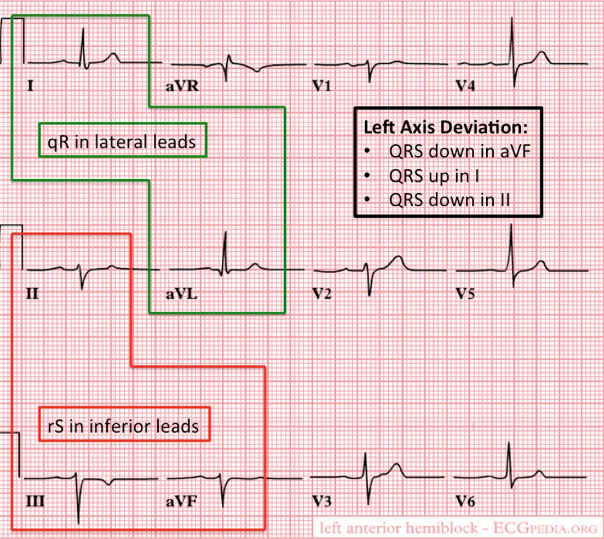
- Criteria for left anterior fascicular block
- QRS normal to slightly prolonged
- Left axis deviation WITHOUT other reasons
- Small R-wave and large S-wave in inferior leads (II, III, aVF)
- Small Q-wave with large R-wave in lateral leads (I, aVL)
- Pathology
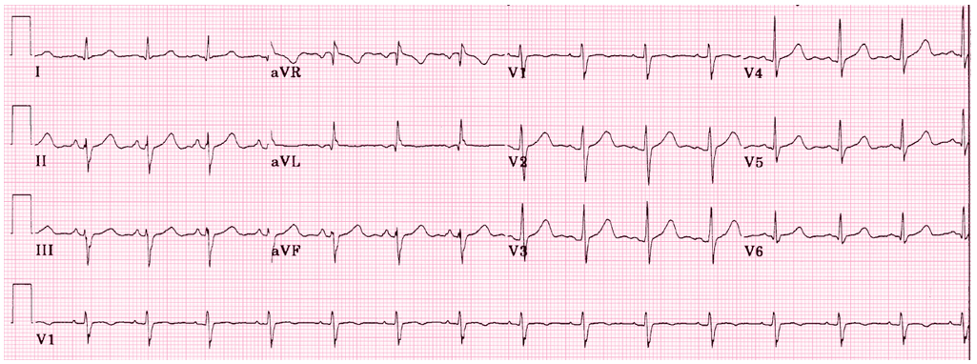
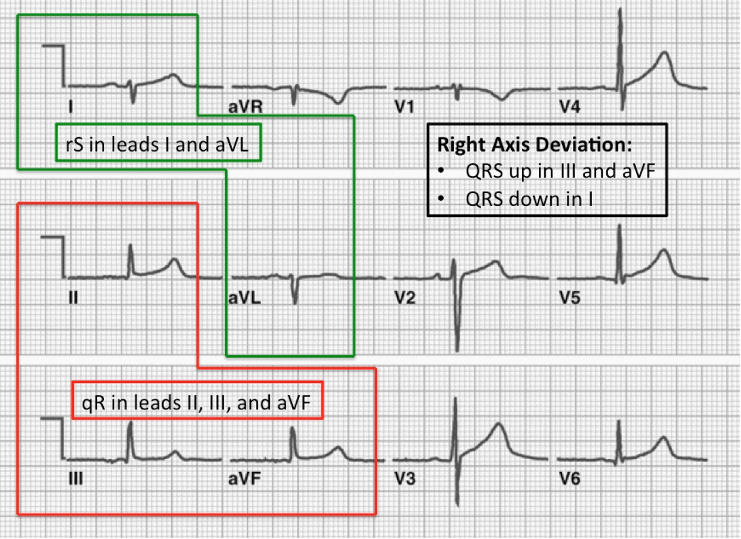
- Posterior
- Pathology
- When blocked, the conduction to the inferior portion of the ventricle is delayed
- Spreads to the intact anterior fascicle and RBB
- Causes right axis deviation
- Spreads to the intact anterior fascicle and RBB
- When blocked, the conduction to the inferior portion of the ventricle is delayed
- Criteria for left posterior fascicular block
- QRS normal to slightly prolonged
- Right axis deviation WITHOUT other reasons
- Small R-wave and large S-wave in lateral leads (I, aVL)
- Small Q-wave and large R-wave in inferior leads (II, III, aVF)
- Pathology
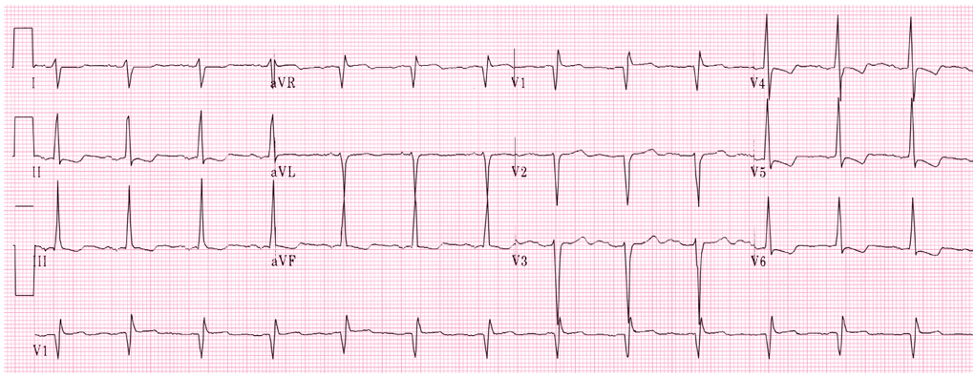
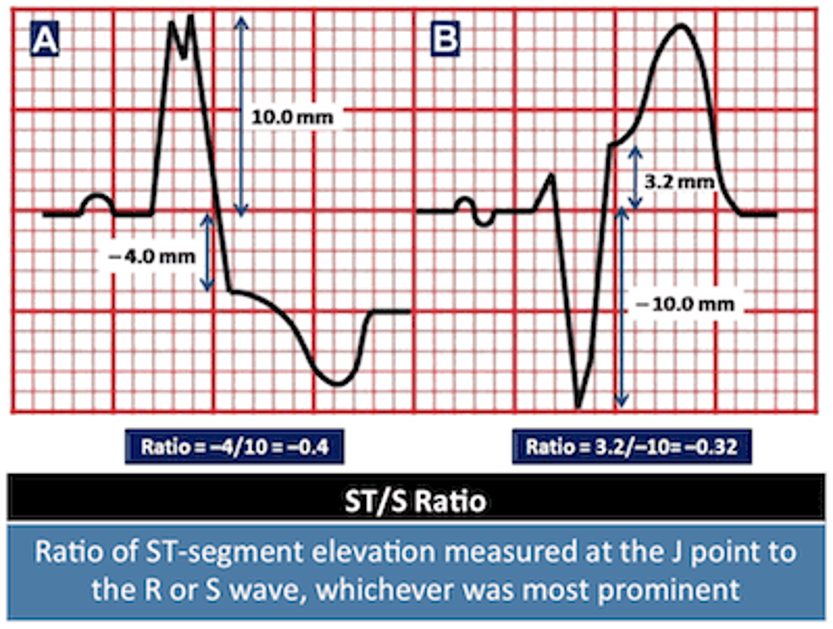
Sgarbossa’s Criteria
- Used in the presence of LBBB or paced rhythm to uncover potential ischemia
- Original (1996)
- Concordant ST elevation > 1mm in leads with a positive QRS complex (5 points)
- Concordant ST depression > 1mm in V1-V3 (3 points)
- Excessively discordant ST elevation > 5mm in leads with a negative QRS complex (2 points)
- Score ≥ 3 has a specificity of 90% for detecting concomitant ischemia
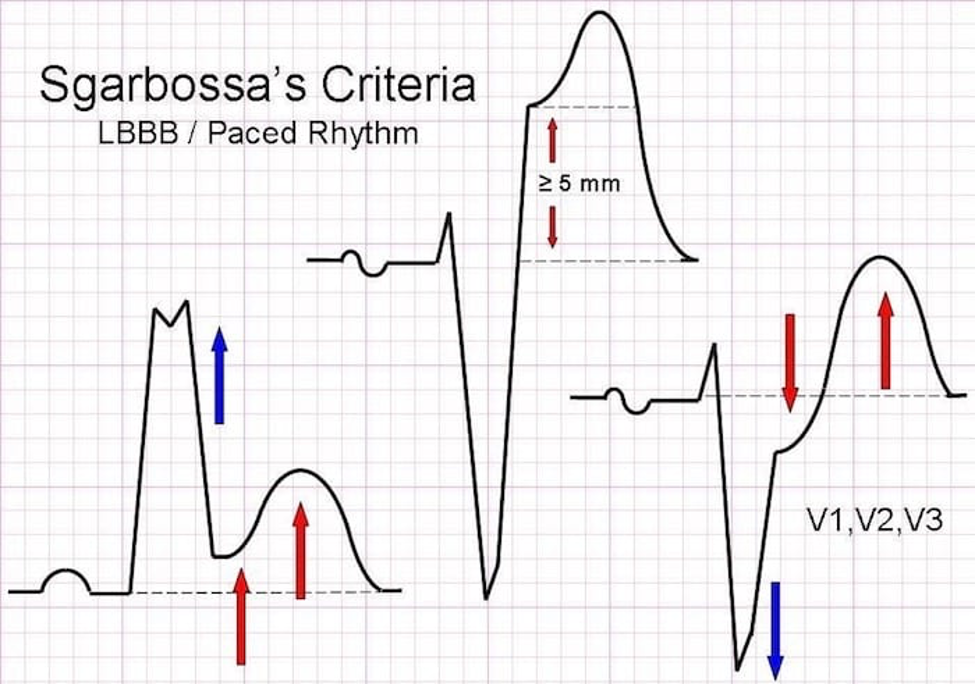
- Smith-Modified Sgarbossa Criteria (2012)
- ≥ 1 lead with ≥ 1mm of concordant ST elevation
- ≥ 1 lead of V1-V3 with ≥ 1mm of concordant ST depression
- ≥ 1 lead ANYWHERE with ≥ 1mm ST elevation AND proportionally excessive discordant ST elevation
- Defined as ≥ 25% of the depth of the preceding S-wave
1893 Cottage Physician

References
- Heart. In: Morton DA, Foreman K, Albertine KH. eds. The Big Picture: Gross Anatomy, 2e. McGraw-Hill; Accessed January 17, 2021.
- Jaffar A. Anatomical Structure of the Heart. In: Elmoselhi A. eds. Cardiology: An Integrated Approach. McGraw-Hill; Accessed January 17, 2021.
- Goldberger AL. Electrocardiography. In: Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J. eds. Harrison’s Principles of Internal Medicine, 20e. McGraw-Hill; Accessed January 17, 2021.
- LITFL. Right bundle branch blocks. https://litfl.com/right-bundle-branch-block-rbbb-ecg-library/
- LITFL. Left bundle branch blocks. https://litfl.com/left-bundle-branch-block-lbbb-ecg-library/
- REBELEM. Bundle Branch Blocks. https://rebelem.com/bundle-branch-blocks101/
- Sgarbossa EB, Pinski SL, Barbagelata A, et al. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. GUSTO-1 (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) Investigators. N Engl J Med. 1996; 334(8):481-7. [pubmed]
- Smith SW, Dodd KW, Henry TD, Dvorak DM, Pearce LA. Diagnosis of ST-elevation myocardial infarction in the presence of left bundle branch block with the ST-elevation to S-wave ratio in a modified Sgarbossa rule. Ann Emerg Med. 2012; 60(6):766-76. [pubmed]
- Meyers HP, Limkakeng AT Jr, Jaffa EJ, et al. Validation of the modified Sgarbossa criteria for acute coronary occlusion in the setting of left bundle branch block: A retrospective case-control study. Am Heart J. 2015; 170(6):1255-64. [pubmed]
- CORE EM. Validation of Modified Sgarbossa Criteria. https://coreem.net/journal-reviews/modified-sgarbossa-criteria/
#67 – Epistaxis
***LISTEN TO THE PODCAST HERE***
Anatomy
Anterior
- Kiesselbach’s Plexus (Little’s area)
- Confluence of three main vessels
- Septal branch of the anterior ethmoidal artery
- Lateral nasal branch of the sphenopalatine artery
- Septal branch of the superior labial branch of the facial artery
- Confluence of three main vessels
Posterior
- Woodruff’s Plexus
- Posteriorlateral branches of the sphenopalatine artery
- Posterior inferior turbinate
- Posteriorlateral branches of the sphenopalatine artery

Epidemiology
- Up to 60% of population will experience a significant nosebleed each year
- Only 10% need to seek attention
- Common ENT admission condition, but rarely needs surgical intervention
- Bimodal age distribution
- Before 10 years or between 45-65 years
- Male predominance before the age of 49, then equalizes
- Estrogen has been shown to protective for mucosa
- Anterior bleeds are significantly more common (>90%) and resolve with minor interventions
- Posterior bleeds can result in significant hemorrhage
Etiologies
- Nose picking
- Low environmental moisture
- Mucosal hyperemia of viral or allergic rhinitis
- Trauma
- Foreign body
- Anticoagulation
- Coagulopathies
- Osler-Weber-Rendu, von Willebrand, hemophilias
- Connective tissue disease
- Aneurysm development
- Neoplasm
- Squamous cell, inverted papilloma
- Hypertension
- Debated as a cause, but has shown to prolong bleeding
- Nasal medications
- Steroids, oxymetazoline
- Heart failure
Patient Assessment
- Primary
- Airway assessment
- RR, O2
- Cardiovascular stability
- HR, BP
- Airway assessment
- Secondary
- History
- Medications
- Anticoagulation, aspirin, nasal medications
- PMH
- Bleeding disorders, HTN, liver disease
- Recent trauma
- History of nosebleeds
- How often, how long do they last, ever been admitted for one
- Medications
- History
- Diagnostic Studies
- Coagulation studies should NOT be routinely ordered
- Should be in patients on anticoagulation
- In patients with prolonged bleeds:
- CBC
- Type and cross
- Coagulation studies should NOT be routinely ordered
- Examination
- Have patient blow nose to remove clots and blood
- Examine nasal cavity to see if you can see the bleeding site
- Otoscope, nasal speculum
- Don’t have patient tilt head back
- Nasopharynx lies in anteroposterior plane and this will obscure the majority of the cavity from view
Interventions
- Initial (Woodpecker/Walrus technique)
- Have patient blow nose to remove clots
- In a small basin mix any or all of the following:
- Oxymetazoline
- Lidocaine with epinephrine
- Tranexamic acid
- If available, soak GelFoam/Surgicel in this fluid and place BEFORE the sponge sticks
- Trim two oral sponge swabs to better fit in the nasal cavity and soak in the fluid
- Make a nasal bridge clamp by taping two tongue depressors together on one end
- Place swabs in nasal cavities and apply nasal clamp for 10-15 minutes
- Ice pack can also be used
- Cautery
- If the bleeding site can be visualized on direct examination
- Apply topical anesthetic
- Silver nitrate sticks
- Start from periphery and roll to center of bleeding
- No more than 10 seconds
- A white eschar should form

- Nasal packing
- Use if cautery fails
- Ensure topical anesthesia
- Soak in sterile water
- Insert by sliding along the floor of the nasal cavity PARALLEL to floor
- Insufflate the balloon with air
- Nasal Balloon Catheters
- For posterior bleeds
- Follow same steps for nasal packing
- Insufflate posterior balloon FIRST and apply gently traction
- Then insufflate the anterior balloon
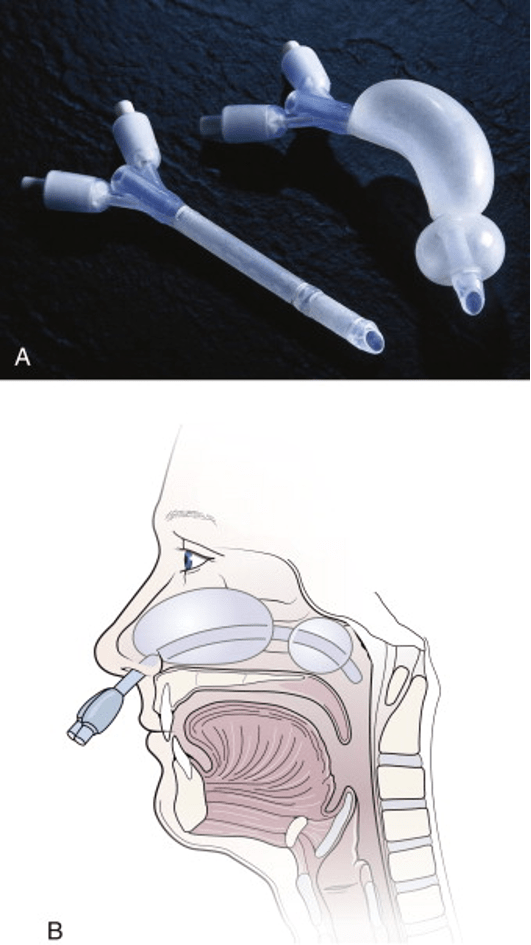
- Foley Catheters
- If you don’t have a prefabricated nasal balloons, a foley catheter can work
- Insert the catheter until you can see it in the posterior oropharynx
- Insufflate with 5-10cc of water
- Apply traction to seat balloon in posterior choana
- Add additional water to tamponade
- Clamp catheter with umbilical clamp or c-clamp from NG tube

Disposition and Follow-up
- For simple nasal packing, patients should be evaluated by ENT within 24-48 hours
- Discuss with consultant need for antibiotic prophylaxis
- No good evidence supports routine use, but ENT often prefers
- Amoxicillin-Clavulanate is most commonly used
- Clindamycin or trimethoprim/sulfamethoxazole should be used if concern for nasal carrier of MRSA
- No good evidence supports routine use, but ENT often prefers
- Discuss with consultant need for antibiotic prophylaxis
- Posterior bleeds should be immediately assessed by ENT for potential surgical intervention
- Endoscopic sphenopalatine artery ligation
- Anterior ethmoid artery ligation
- Open or endoscopic

1893 Cottage Physician

References
- Kucik CJ, Clenney T. Management of epistaxis. Am Fam Physician. 2005; 71(2):305-11. [pubmed]
- Villwock JA, Jones K. Recent Trends in Epistaxis Management in the United States JAMA Otolaryngol Head Neck Surg. 2013; 139(12):1279-84. [pubmed]
- Kotecha B, Fowler S, Harkness P, Walmsley J, Brown P, Topham J. Management of epistaxis: a national survey. Ann R Coll Surg Engl. 1996; 78(5):444-6. [PDF]
- Fishpool SJ, Tomkinson A. Patterns of hospital admission with epistaxis for 26,725 patients over an 18-year period in Wales, UK. Ann R Coll Surg Engl. 2012; 94(8):559-62. [PDF]
- Min HJ, Kang H, Choi GJ, Kim KS. Association between Hypertension and Epistaxis: Systematic Review and Meta-analysis. Otolaryngol Head Neck Surg. 2017; 157(6):921-927. [pubmed]
- Shakeel M, Trinidade A, Iddamalgoda T, Supriya M, Ah-See KW. Routine clotting screen has no role in the management of epistaxis: reiterating the point. Eur Arch Otorhinolaryngol. 2010; 267(10):1641-4. [pubmed]
- Lin G, Bleier B. Surgical Management of Severe Epistaxis. Otolaryngol Clin North Am. 2016; 49(3):627-37. [pubmed]
#66 – How to be a Good Student on Emergency Medicine Rotation
***LISTEN TO THE PODCAST HERE***
Guest for the Episode
John B. Hurt, MPAS, PA-C

My 10 Rules
- Be on time
- On-time = early
- Introduce yourself to the team
- Preceptor and/or supervising physician
- Charge nurse
- Secretary or unit clerk
- Be Goal Oriented
- Shift
- Have 1-2 objectives for every shift you work
- Rotation
- Talk with your preceptor about what you want to see/do/experience
- Actively seek out these experience
- Talk with your preceptor about what you want to see/do/experience
- Shift
- Always Be Doing Something
- Checking labs/images
- Ask to draw blood for techs
- Ask if you can get anything for your preceptor/staff
- Review cases
- There is something to be learned about every case we see in the ED
- Look them up, write them down
- Ask your preceptor what their decision making looks like
- Develop an algorithmic approach to common ED presentations
- Chest pain, abdominal pain, AMS, fever, dyspnea, back pain, nausea/vomiting, trauma
- Fast the providers what they do
- Write these out to reference in the future
- Get familiar with clinical decision instruments
- Ottawa Rules, NEXUS, PERC, Canadian C-Spine
- Chest pain, abdominal pain, AMS, fever, dyspnea, back pain, nausea/vomiting, trauma
- 1-minute Presentations are an absolutely must
- Have your diagnosis and plan ready
- Differential diagnosis
- Life threats, most likely, plausible, Zebras
- Disposition is the end decision in EM
- Every patient either gets admitted or discharged
- Before your preceptor makes the decision, make your own and see if your right
- See every type of patient that walks through the door
- Don’t cherry pick (unless it is on your bucket list)
- This will help you immensely throughout your career
- Go up and see the patients you admit before you leave the hospital after a shift
- It will benefit your decision in the long run if you see what the inpatient team is doing
- Your patients will also appreciate you checking on them
- Get feedback after every shift (if possible)
- Plus-Delta approach
- Things you do well (plus)
- Things you can change (delta)
- Plus-Delta approach
PAINE #PANCE Pearl – Cardiology
Question
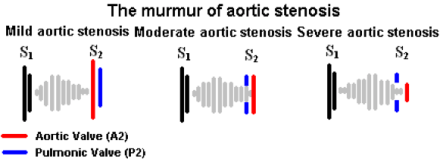
73yo man, with a history of hypertension and coronary disease, is brought into the emergency room after a witnessed syncopal episode at home. He reported some mild exertional chest pain over the past few days, but states that it improved with rest. Vital signs are BP-180/98, HR-74, RR-12, and O2-100%. He is currently in no distress and not diaphoretic. Physical examination revealed a systolic murmur over the 2nd right intercostal space. A CT was ordered to rule-out PTE in the setting of chest pain and syncope and is below, along with the murmur.
- What is the diagnosis?
- How would you describe this murmur?
- Where would you expect this murmur to radiate?
- What is the classic triad associated with this condition?
Answer
- Aortic Stenosis due to a calcified aortic valve
- High-pitched, crescendo-decrescendo (diamond shaped), midsystolic, ejection murmur with a soft S2
- AS murmurs transmit well and equally to the carotid arteries
- The classic triad of AS is exertional angina, exertional dyspnea, and dizziness/syncope

PAINE #PANCE Pearl – Cardiology
Question
73yo man, with a history of hypertension and coronary disease, is brought into the emergency room after a witnessed syncopal episode at home. He reported some mild exertional chest pain over the past few days, but states that it improved with rest. Vital signs are BP-180/98, HR-74, RR-12, and O2-100%. He is currently in no distress and not diaphoretic. Physical examination revealed a systolic murmur over the 2nd right intercostal space. A CT was ordered to rule-out PTE in the setting of chest pain and syncope and is below, along with the murmur.
- What is the diagnosis?
- How would you describe this murmur?
- Where would you expect this murmur to radiate?
- What is the classic triad associated with this condition?
PAINE #PANCE Pearl – Dermatology
Question
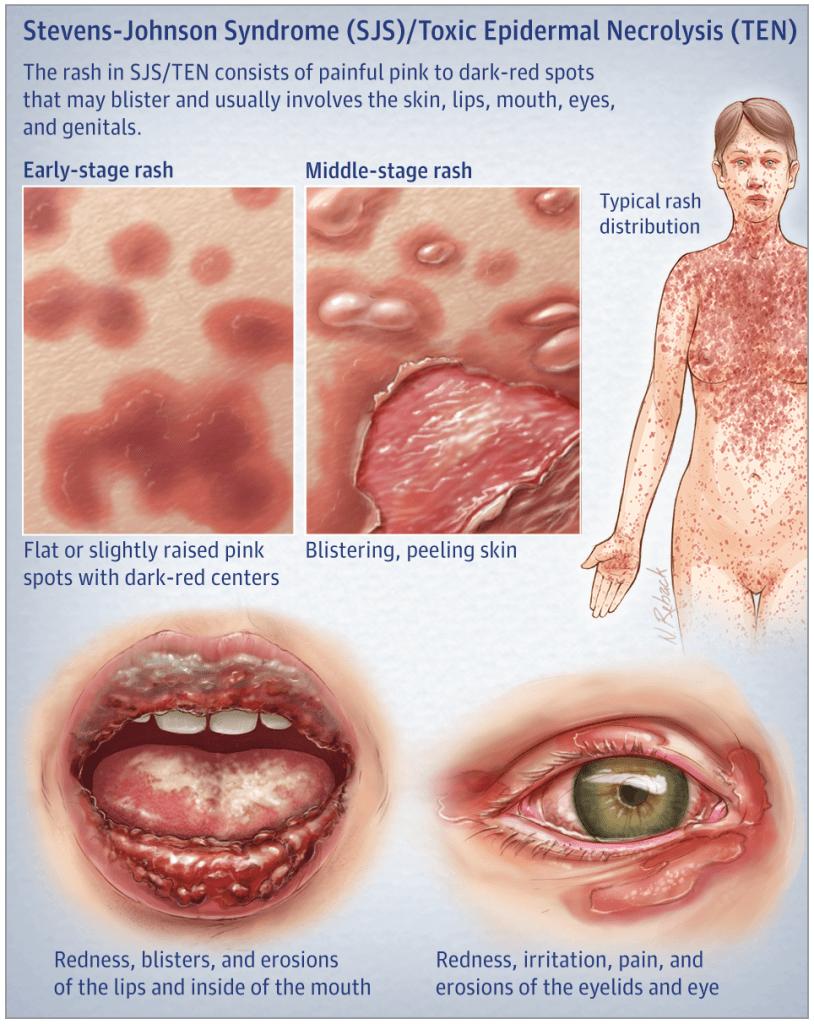
A 29yo patient is seen for a severe drug reaction after starting lamotrigine (Lamictal) for new-onset epilepsy. She has significant desquamation of her mucous membranes as well as large patches of denuded epidermis with multiple bullae present.
- What is the clinically distinguishing feature between Steven-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)?

Answer
The main clinical difference between SJS and TEN is the severity and degree of involvement. SJS classically is < 10% TBSA involvement, where as TEN is > 30% TBSA.

PAINE #PANCE Pearl – Dermatology
Question
A 29yo patient is seen for a severe drug reaction after starting lamotrigine (Lamictal) for new-onset epilepsy. She has significant desquamation of her mucous membranes as well as large patches of denuded epidermis with multiple bullae present.
- What is the clinically distinguishing feature between Steven-Johnson Syndrome and Toxic Epidermal Necrolysis?

Ep-PAINE-nym
Le Fort Fractures
Other Known Aliases – transfacial fracture of the midface
Definition – These fractures involve the maxillary bone and are graded based on their direction and involvement of surrounding structures. The key distinguishing feature of this type of fracture is separation of the pterygoid plates from the maxillary sinuses.
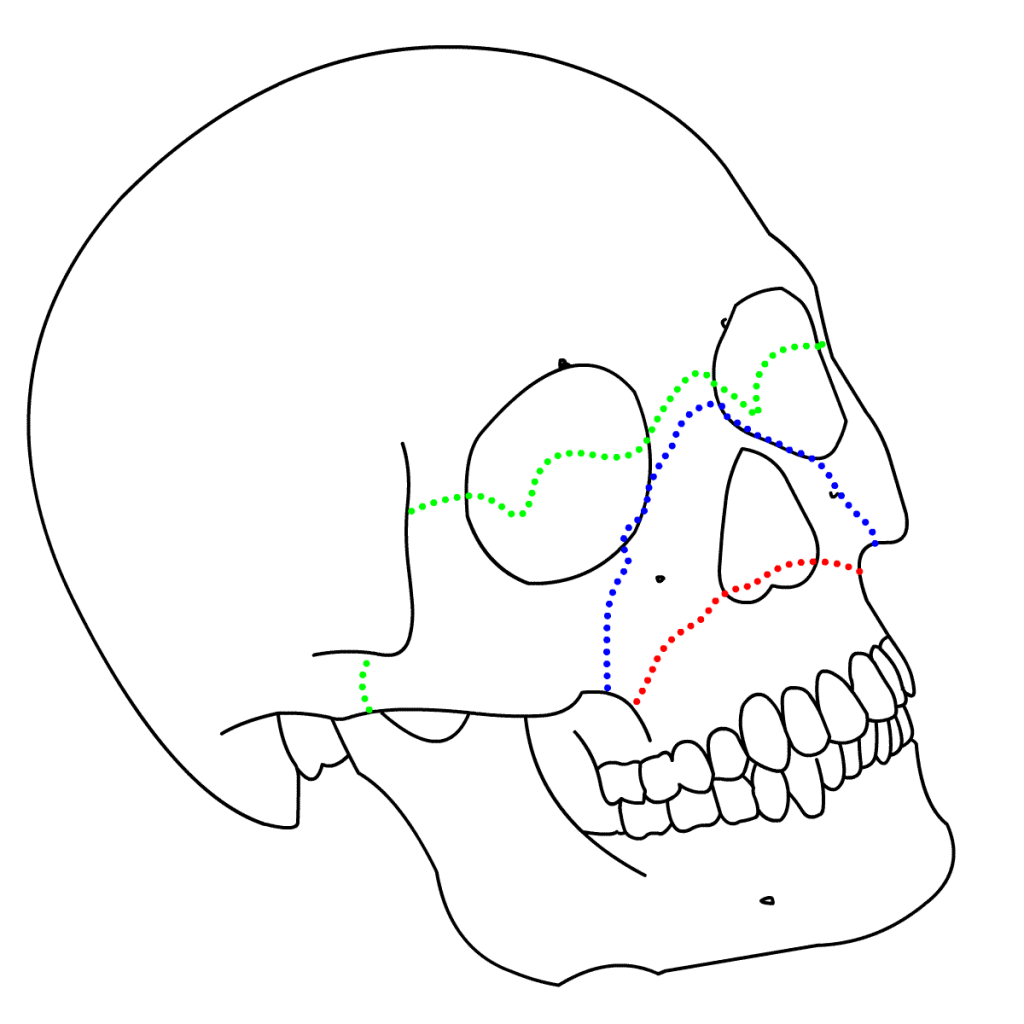
Clinical Significance – Continuity of the pterygoid plates is essential for midface structural stability and any disruption requires surgical fixation. There are three types of Le Fort fractures:
- Type I – Horizontal fracture – involves the lateral bony margin of the nasal opening
- Type II – Pyramidal fracture – involves the inferior orbital rim
- Type III – Transverse fracture – involves the zygomatic arch, vomer, and across the orbital floor and walls

History – Named after René Le Fort (1869-1951), who was a French surgeon and received his medical doctorate at the age of 21 while serving in the French military. He taught and practice in Lille, France for the majority of his career. He served his country numerous times when called to serve as a military physician, as well as coming out of retirement during World War II to teach at the University of Lille to replace colleagues called to the war effort. He published the findings of his eponymous conditions in 1901 in a treatise entitled “Étude expérimentale sur les fractures de la mâchoire supérieure”, where he described his experiments of dropping cannon balls from varying directions and heights on the faces of cadavers to describe the predictable injury patterns

References
- Firkin BG and Whitwirth JA. Dictionary of Medical Eponyms. 2nd ed. New York, NY; Parthenon Publishing Group. 1996.
- Bartolucci S, Forbis P. Stedman’s Medical Eponyms. 2nd ed. Baltimore, MD; LWW. 2005.
- Yee AJ, Pfiffner P. (2012). Medical Eponyms (Version 1.4.2) [Mobile Application Software]. Retrieved http://itunes.apple.com.
- Whonamedit – dictionary of medical eponyms. http://www.whonamedit.com
- Up To Date. www.uptodate.com
- Gartshore L. A brief account of the life of René Le Fort. The British journal of oral & maxillofacial surgery. 2010; 48(3):173-5. [pubmed]
- Patterson R. The Le Fort fractures: René Le Fort and his work in anatomical pathology. Canadian journal of surgery. Journal canadien de chirurgie. 1991; 34(2):183-4. [pubmed]
- Le Fort R. Étude expérimentale sur les fractures de la machoire supérieure. Revue de chirurgie, Paris 1901; 23: 208-27; 360-79; 479-507
PAINE #PANCE Pearl – Critical Care
Question
A large part of critical care and ICU management revolves around hemodynamic monitoring and support. But…..we typically don’t use traditional blood pressure (systolic and diastolic) numbers directly.
We use MAP!!!
- What is MAP?
- How do you calculate it?
- Why is it a better variable to monitor when it comes to blood pressure and critical care?
Answer
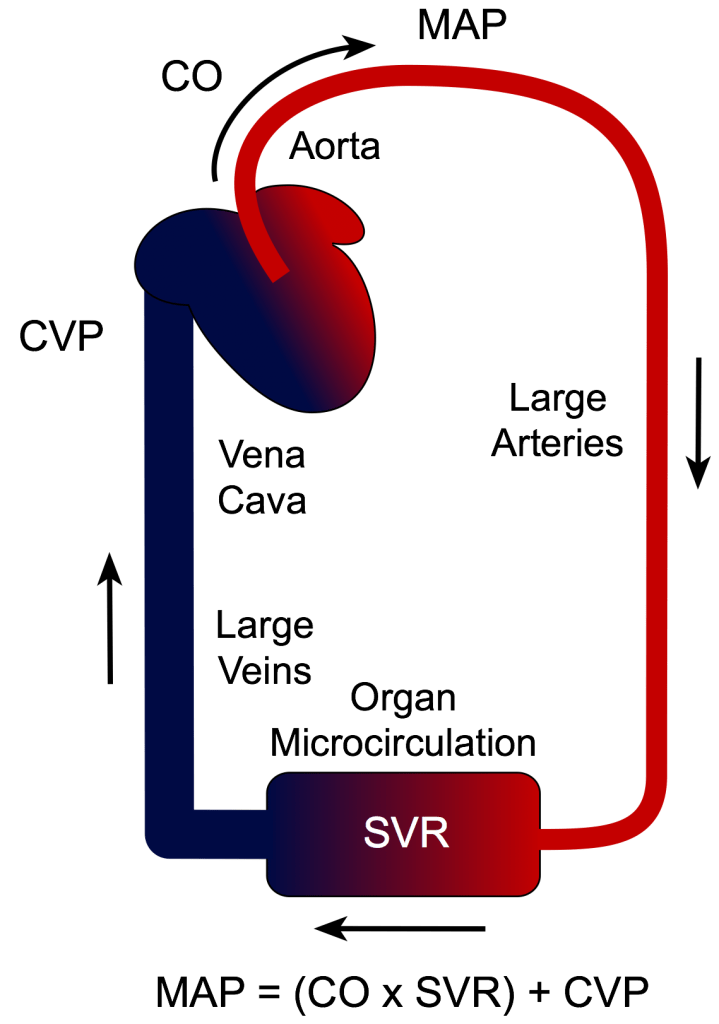
- Mean Arterial Pressure (MAP)
- It is calculated using the following formula:
- MAP = 1/3(SBP) + 2/3(DBP)
- MAP has the greatest influence on blood flow autoregulation within the organs, as well as whole body hemodynamic homeostasis. It is superior to systolic pressure because it is the true driving pressure for peripherial blood flow and it does not change as the pressure waveform moves more distally.
- Bonus Pearl – MAP > 65 is a general ICU mantra as the minimum pressure pressure to maintain organ perfusion


References










